by Linnea Wahl, MS, Team 5, Daily Clout:
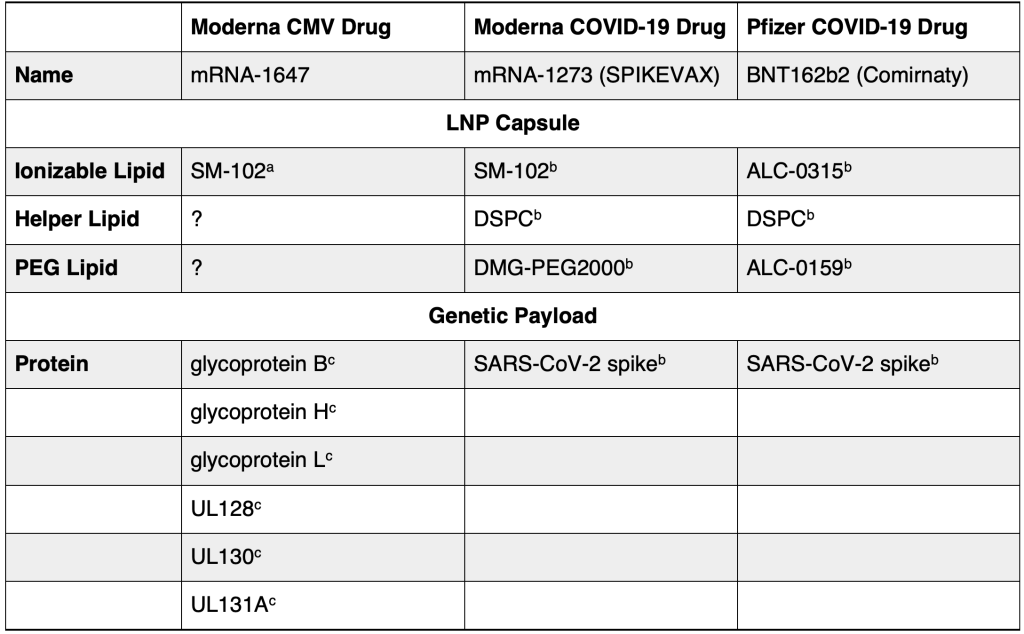
Moderna researchers did not test their COVID-19 mRNA drug, called SPIKEVAX, to find out where it would go in our bodies (biodistribution). Instead, their biodistribution study was for a completely different vaccine. Despite this substitution of one drug study for another, the U.S. Food and Drug Administration (FDA) approved SPIKEVAX for both emergency and routine use by Americans.
Introduction
The FDA declared in its own words that Moderna researchers did not test their COVID-19 mRNA drug SPIKEVAX to determine its distribution throughout the body. In the FDA reviewers’ own words: “A biodistribution study was not performed with mRNA-1273 vaccine [SPIKEVAX].



