by Carol Taccetta, Daily Clout:
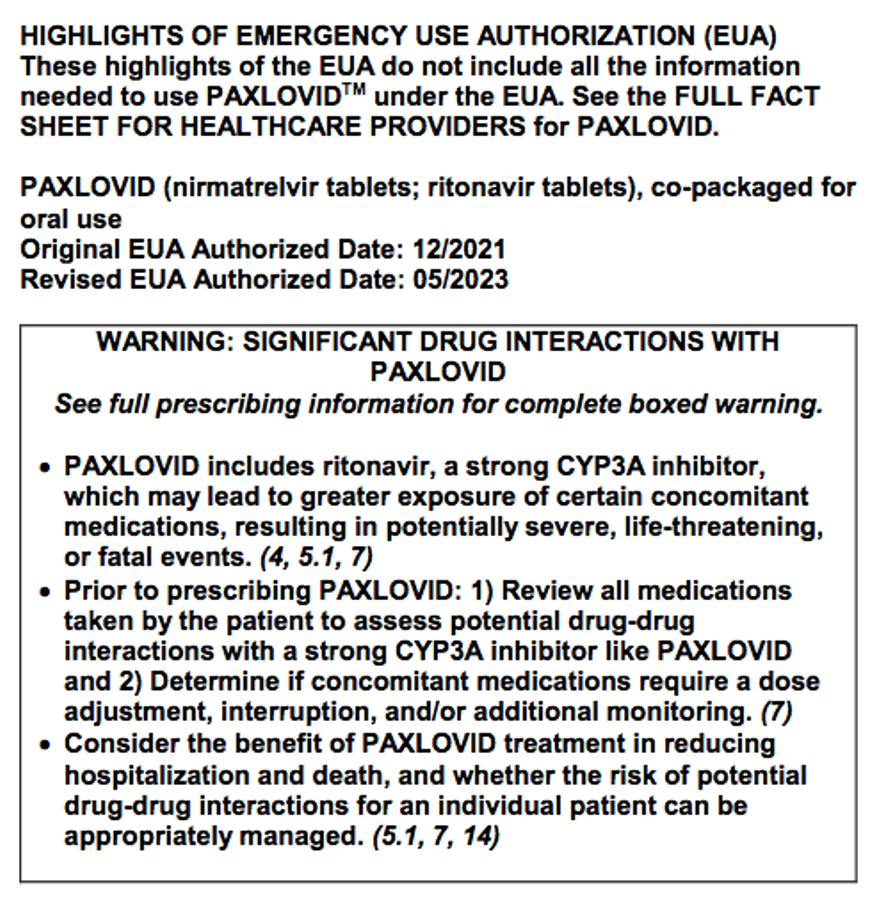
There are some adverse reactions in a product label that are so serious, and possibly even preventable, that special labeling is required to highlight this warning information: a “BOXED WARNING.”

https://www.fda.gov/media/71866/download
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=201.57
This boxed warning information, “critical for a prescriber to consider,” is commonly referred to as a “black box.” (https://www.fda.gov/media/71866/download)



