by Carol Taccetta, Daily Clout:
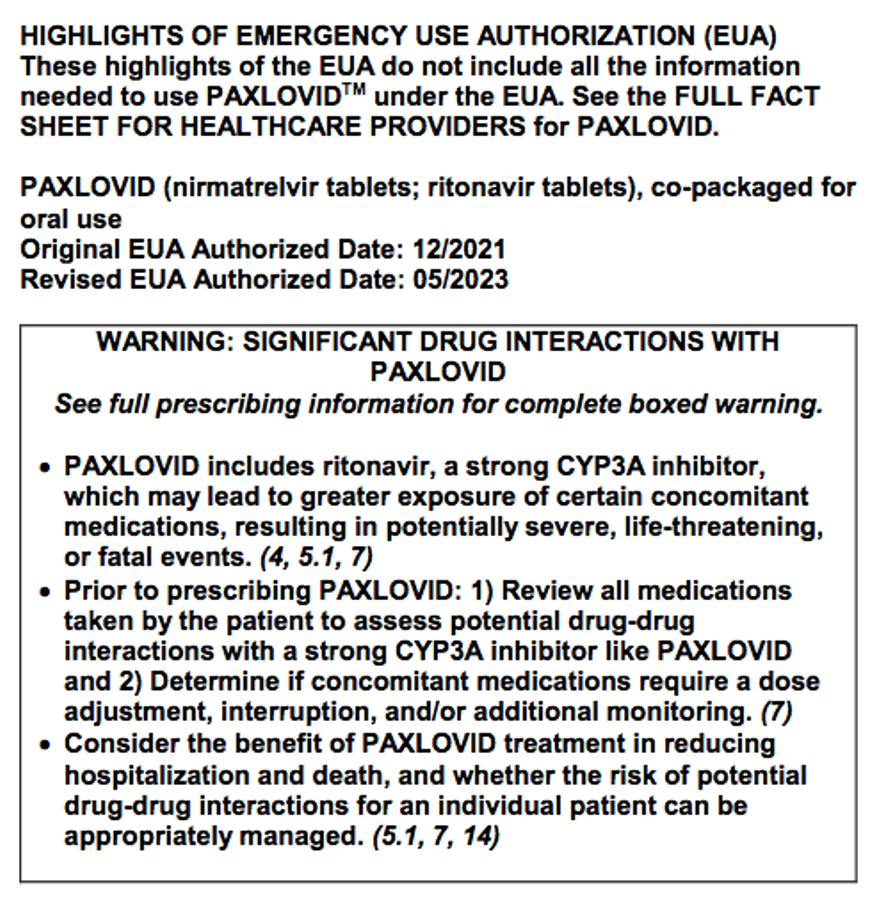
There are some adverse reactions in a product label that are so serious, and possibly even preventable, that special labeling is required to highlight this warning information: a “BOXED WARNING.”

https://www.fda.gov/media/71866/download
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=201.57
This boxed warning information, “critical for a prescriber to consider,” is commonly referred to as a “black box.” (https://www.fda.gov/media/71866/download)
TRUTH LIVES on at https://sgtreport.tv/
Boxed Warnings are absent from the labeling of all three currently available COVID-19 vaccines in the United States. In the letter below, I write to the Food and Drug Administration (FDA), requesting that certain CONTRAINDICATIONS and WARNINGS AND PRECAUTIONS “rise to the level of a Boxed Warning.” (https://www.fda.gov/media/71866/download and https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/labeling-changes-mortality-kidney-injury-and-excess-bleeding-hydroxyethyl-starch-products)
As an aside: During my research, I noted that, despite their differing platforms (mRNA vs. protein subunit-based), all three vaccines share the same five warnings, suggesting a common denominator, such as being spike-protein based. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html)
Dr. Taccetta’s Letter to Dr. Peter Marks, Director of CBER at the U.S. FDA
July 23, 2023
Peter Marks, MD, PhD
Director, Center for Biologics Evaluation and Research
U.S. Food and Drug Administration
10903 New Hampshire Avenue
W071-7232
Silver Spring, MD 20993
mailto:Peter.Marks@fda.hhs.gov
Dear Dr. Marks,
Based on my experience of working with the FDA for 25+ years in drug safety, and in light of the FDA’s own guidance of 2011, I interpret the COVID-19 vaccine warning information under CONTRAINDICATIONS, as well as “Management of Acute Allergic Reactions” and “Myocarditis and Pericarditis” under WARNINGS AND PRECAUTIONS as all rising to the level of a Boxed Warning. (https://www.fda.gov/media/71866/download)
Please note: In my assessment, there are many other “observed serious adverse reactions” which are not mentioned in the current product labeling: my arguments below are solely based on the information currently acknowledged by the FDA in the Fact Sheet Labeling (and its hyperlinks) as a stand-alone document.
The Boxed Warning guidance applies to emergency use authorization (EUA) products, as well as approved products, as seen in this recently revised EUA Paxlovid Fact Sheet:

https://labeling.pfizer.com/ShowLabeling.aspx?id=16474&format=pdf
Today, there are three COVID-19 vaccines available in the U.S., each under EUA only:
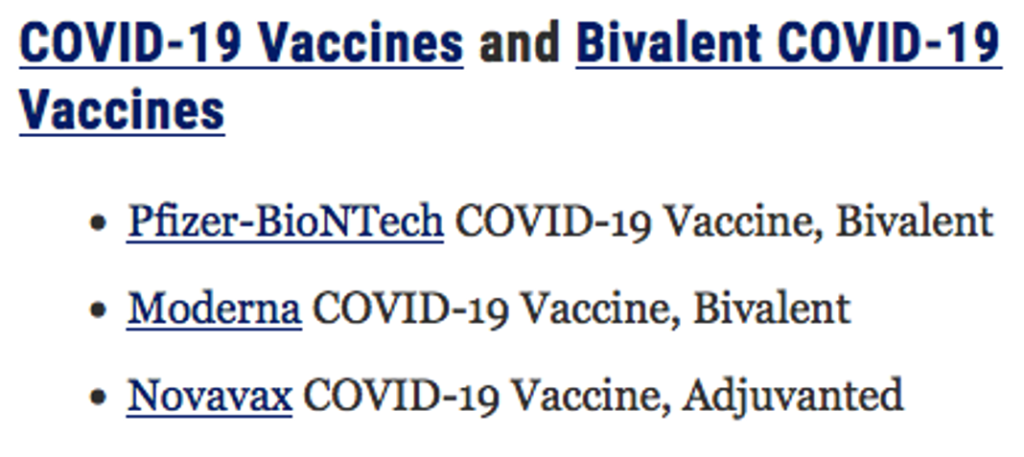
Under Section 4, CONTRAINDICATIONS, each manufacturer contraindicates use of their respective vaccine in individuals with a known history of severe allergic reaction to a component in their own vaccine. Also, each fact sheet carries the same five warnings under Section 5, WARNINGS AND PRECAUTIONS: 5.1 Management of Acute Allergic Reactions, 5.2 Myocarditis and Pericarditis, 5.3 Syncope, 5.4 Altered Immunocompetence and 5.5 Limitations of Vaccine Effectiveness. (https://www.fda.gov/media/167211/download, https://www.fda.gov/media/167208/download, and https://www.fda.gov/media/159897/download)
As you know, a “Boxed Warning” should appear in the product’s label if warning information under WARNINGS AND PRECAUTIONS or CONTRAINDICATIONS meets one of the following criteria:



